Sep. 16, 2020
Although the COVID-19 pandemic was in decline in the summer of 2020, it struck again with the beginning of Autumn. Numbers of infected are growing worldwide. The United States, Turkey, Europe and India are watching the growth of coronavirus infections with alarm.
At the same time, the development of vaccines which could provide general immunity are reaching the finish line. These vaccines are different, created by different pharmaceutical companies and supported by different countries.
In the context of the COVID-19 pandemic and its social and political consequences, vaccines are themselves becoming a geopolitical tool. They testify to the strength of the state, its scientific potential, its ability to organize complex work, and to conduct effective and sovereign biopolitics.
A COVID-19 vaccine will help secure a country’s sovereignty in the post-covidal world and ensure claims to leadership in the emerging multipolar world.
According to Johns Hopkins University, the number of detected cases of coronavirus infection around the world has exceeded 27 million 880,000 – more than 900,000 people have died from complications developed against the background of coronavirus infection and related diseases.
Russia’s “Sputnik” – the first vaccine
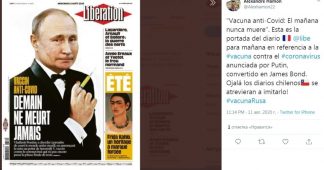
On August 11, at a meeting with government members on Tuesday Russian President Vladimir Putin said that the world’s first coronavirus vaccine had been successfully tested in Russia.
The vaccine is called “Sputnik V”, and it is a vector two-component adenovirus-based vaccine. Vector in this case means that for delivery of genetic information SARS-CoV-2 to the human body a carrier based on another virus (vector) is used, and that the two components come from two separate preparations (based on two slightly different vectors-adenoviruses). People are immunized in two stages with an interval of three weeks.
A number of countries have defiantly refused the Russian vaccine, such as Ukraine. On the other hand, in Belarus, which is in a difficult situation of mass protests, the decision to adopt the Russian vaccine has become one of the symbols of its geopolitical alliance with Moscow.
Mexico and Kazakhstan have already signed agreements with Russia to supply the vaccine. Venezuela and Uzbekistan are also exploring this possibility.
However, Moscow refuses to supply foreign vaccines against COVID-19.
On September 7, a group of scientists from different countries (first of all, the US and Italy) published an open letter to The Lancet magazine and the authors of an article on Russian vaccine trials, where they questioned the effectiveness of the Russian development.
Turkey may soon allow testing of Russian coronavirus vaccine on its territory, Turkish Health Minister Dr. Fahrettin Koca said on September 2.
Turkey: a claim to sovereignty
Turkey is already testing two vaccines of its own on volunteers. In August, Dr. Fahrettin Koca reported that local scientists are developing thirteen vaccines against coronavirus SARS-CoV-2. Three of these vaccines have already been successfully tested on animals.
Meanwhile, the Turkish Minister of Health reported that Moscow and Ankara have agreed to collaborate on cross-clinical trials of their vaccines. The Minister previously reported on joint work that Turkey is doing in this area with China and Germany.
According to officials (Professor Hasan Mandal, head of the Scientific and Technological Research Council of Turkey (TÜBİTAK), the Turkish vaccine will be available to the public in early 2021.
China – Vaccine diplomacy
While Russia claims to be the first country to register a vaccine against the novel coronavirus, China is challenging this claim.
On June 29, Chinese biotechnology company CanSinoBio received permission to use the vaccine based on adenovirus among the Chinese military.
In total, eight COVID-19 vaccines are being developed in China.
Recently, the State Food and Drug Administration of China approved the clinical trial of the developed vaccine against coronavirus infection in the form of nasal spray. According to the Xinhua News Agency, this is the first COVID-19 spray vaccine approved in China for human clinical trials.
On September 7, pharmaceutical companies – Sinovac Biotech, China National Biotec Group (CNBG) and Sinopharm – presented potential Chinese coronavirus vaccines COVID-19 for the first time.
In May, President Xi Jinping promised that in that case of successful testing, the vaccines developed by China will become a “global public good”.
Participants in the third phase of Chinese vaccine trials: United Arab Emirates, Bahrain, Peru, Morocco, Argentina, Turkey, Bangladesh, Serbia, Pakistan, Indonesia and Jordan.
China is actively working with the WHO through the COVAX initiative, which aims to ensure access to vaccines for all countries in the world. The US officially refuses to participate in this initiative.
However, the White House believes that the WHO compromised itself by negligently or intentionally misrepresenting Covid-19 data from China at the beginning of the pandemic. The Trump administration says that the US will continue to work closely with international partners in the fight against coronavirus, but not as part of a project controlled by the “corrupt WHO” and China.
US: Trump’s strategy at risk
According to US Secretary of State Mike Pompeo, Russia and China rushed to market their coronavirus vaccines not to make a medical or epidemiological breakthrough, but to make a political and diplomatic breakthrough.
Pompeo’s statement came after the Swedish-British pharmaceutical company AstraZeneca announced the suspension of phase three of its vaccine trials due to side effects on one of the test subjects. According to preliminary data, one of the test subjects was found to have a neurological symptom similar to that of spinal cord inflammation.
Previously, it was the AstraZeneca vaccine that the US authorities pinned their hopes on “winning” the vaccine race. For Donald Trump, finding a possible way to deal with the coronavirus may be an important campaign victory in the attempt to win the US Presidency once again on November 3.
At the same time, Chinese and Russians, making claims about successful trials of such vaccines, make Western scientists and pharmaceutical companies look like losers.
On September 9, White House spokeswoman Kayleigh McEnany said that the US plans to receive the vaccine for coronavirus infection by the end of 2020, despite the suspension of testing by AstraZeneca.
Earlier, US President Donald Trump said that the United States expects to receive the vaccine by the end of October.
The president noted the company Pfizer, which, he said, expects to receive the results of the drug trials in the coming weeks. Johnson & Johnson, Merck and Moderna are also developing a vaccine in the US.
Back in July, the US company Moderna began the third and final stage of coronavirus vaccine trials, which involved 30,000 people. For the creation of the vaccine, Moderna received $483 million from the Department of Biomedical Research of the US Department of Health.
The US Department of Health has instructed all 50 states to prepare for a possible vaccination of part of the population already on November 1 – two days before the presidential election. US chief epidemiologist Anthony Fauci did not rule out that vaccination may begin even before the full completion of tests.
US voters are extremely sceptical about the vaccines for the new coronavirus that are being developed in the country, and most of them believe that the work is being done in an undue hurry. This is evidenced by the results of a sociological survey published on Sunday, conducted in the period from 2 to 4 September by the research service YouGov, 58% of respondents are afraid to get vaccinated.
At the same time black voters are the most suspicious of vaccination, at the same time, thy are the portion of the US population which has been most disproportionately hit by coronavirus
According to the Pew Research Center, only 54% of black adults in the US say they would take an approved COVID-19 vaccine, compared to 74% of white and Hispanic adults.
EU: a multilateral approach
On July 17, the European Commission approved The European Vaccines Strategy, which implies that the EU enters into Advance Purchase Agreements with companies that will supply coronavirus vaccine to Europe.
These are the products of CureVac (Germany), Moderna (USA), BioNTech (Germany) and Pfizer (USA), AstraZeneca (UK-Sweden), Sanofi (France) and GSK (UK) as well as Johnson & Johnson (USA).
The vaccine, developed by BioNTech and Pfizer, may appear on the EU market by the end of 2020.
On September 7, BioNTech and Pfizer announced the final phase of clinical trials in Germany.
Another German company – CureVac, backed by the Bill & Melinda Gates Foundation, is conducting clinical trials.
Klaus Cichutek, head of the Paul Ehrlich Institute, said vaccination of some groups in Germany may begin in the first months of 2021.
India: their own and vaccines, and others’
In August, Indian Prime Minister Narendra Modi stated that India is testing three vaccines against coronavirus infection and a plan to distribute them to citizens is in place.
India’s Minister of Health, Dr. Harsh Vardhaan, said on Sunday, August 23, that he expects to develop a vaccine against coronavirus in the country by the end of this year.
Earlier, two Indian companies reported that they had started the first phases of testing their vaccines. India’s Serum Institute also started the final phase of testing another coronavirus vaccine in the country, developed jointly with Oxford University.
The Russian authorities also do not rule out that the vaccine developed by Russian scientists will be produced in India. A condition of this deal could be the transfer of Russian biotechnology to India.
At the same time, trials of the problem vaccine AstraZeneca and Oxford University have been stopped in India.
Japan: loyalty to the West
The Japanese government decided on Tuesday, September 8, to allocate 671.4 billion yen ($6.3 billion) to purchase COVID-19 vaccines. Vaccines are planned to be purchased from the U.S. and Britain. Japan has already signed contracts with British-Swedish pharmaceutical company AstraZeneca and American Pfizer for the supply of 120 million doses of their experimental vaccines.
Japanese scientists are also developing their own vaccines against coronavirus SARS-CoV-2. However, their commercial sales may start later than their foreign equivalents.
Brazil: The loss of sovereignty
The vaccination of the Brazilian population against coronavirus SARS-CoV-2 may begin in January 2021. That was said by Acting Minister of Health of Brazil Eduardo Pazuello at a meeting of the government.
Earlier, the Brazilian authorities signed an agreement with the Anglo-Swedish pharmaceutical company AstraZeneca Plc. for joint research and production of Oxford vaccine against SARS-CoV-2 in the country.
In addition, Chinese, German and American coronavirus vaccines are being clinically tested in Brazil. The State of Parana earlier announced its intention to start testing the Russian vaccine Sputnik V.
According to the governor of the state of São Paulo João Doria, the Chinese vaccine trials of Sinovac company are still going so well that the first vaccinations may begin in December 2020.
However, no vaccines have been developed in Brazil. The latter is evidence that Brazil no longer claims to be one of the leading players on the world stage. The absence of its coronavirus vaccine means that it is unable to conduct effective biopolitics and protect the health of citizens. Brazil has not passed the sovereignty test, and as well as almost the entire Latin American region can be attributed to countries with limited sovereignty.
Cuba – an island of sovereignty
Cuba is a significant exception. On August 24, the country began the first phase of clinical trials of its own vaccine against coronavirus infection COVID-19. The vaccine is called “Soberana 01” (“Sovereign 01”) and was developed by Cuban State Institute Finlay. The results of the trials will be made public in February 2021.
Cuba may also become one of the places where it will be possible to produce the Russian vaccine starting November this year. Cubans are also working closely with Chinese scientists and physicians to develop the drug from COVID-19.
Cuba has extensive experience in medical research and vaccine production. Cuban healthcare is rightly considered one of the best in the world. During the pandemic, the role of Cuban medical diplomacy increased tremendously. Cuban doctors have gone to provide assistance around the world, which has a positive impact on the country’s position on the world stage and the attitude of the people of countries, especially the Third World, towards the Cuban state and social system.
Africa: testing ground
African countries are in a similar situation. Some of them offer curious ways to fight the coronavirus on the basis of folk remedies – for example, this is how the authorities of Madagascar.
However, many African countries have turned into research sites for testing foreign vaccines. For example, the same vaccine from AstraZeneca.
In April 2020, Dr Tedros Adhanom Ghebreyesus, WHO Director-General, stated that “Africa can’t and won’t be a testing ground for any vaccine”. Previously, two doctors on French TV stated the need to test vaccines for coronavirus in Africa.
However, due to the fact that no African country is creating a sovereign coronavirus vaccine, African continent in fact has become a testing ground for a wide variety of coronavirus vaccines from abroad with South Africa leading the process.
The Globalist Council on Foreign Relations says, “It is appropriate that South Africa host the vaccine trials,” commenting on the start of British Coronavirus vaccine trials in South Africa.
However, there are protests against these trials. Protesters say they were against “the gates of poison,” and Africans are “no Guinea pigs”.
African countries are massively involved in COVAX, a global coronavirus vaccine distribution initiative founded by the Coalition for Epidemic Preparedness Innovations (CEPI), the World Health Organization and GAVI (Global Alliance for Vaccines and Immunization).
GAVI is a global alliance involving some developed countries, the WHO, UNICEF, the World Bank, vaccine developers and manufacturers from different countries, research and technical agencies, community organizations, and foundations including the Bill & Melinda Gates Foundation.
CEPI was launched at the globalist World Economic Forum (WEF) in Davos in 2017 – by Norway, Japan, and Germany, The Wellcome Trust (UK), and the Bill & Melinda Gates Foundation.
However, independent experts have serious questions about the vaccines distributed by the Gates Foundation. Gates is accused of seeking to reduce Africa’s population with vaccines and lobbying Western pharmaceutical companies that supply low-quality or poorly researched vaccines to Third World countries.
The test of sovereignty
In the modern world, the possession of a homegrown coronavirus vaccine is nearly equivalent to full geopolitical sovereignty. It is a proof of the effectiveness of the state, its scientific and health centers, the ability to fight the pandemic in a sovereign manner and to freely build geopolitical alliances. In addition, it is an important tool to promote its influence on the world arena, in those countries that cannot afford their own vaccine.
The main contradictions and antagonisms in vaccine competition are now between China and the United States. Russia confidently claims to be independent and cooperates with China. Thus, it also passed the test for the role of an independent center of power in the world.
But the other two BRICS countries – Brazil and South Africa – have not passed such a test, and are not developing their own vaccines. India looks very unconvincing so far, which coincides with the country’s pro-Western course.
This means that in the post-coronavirus world, these countries’ positions are much weaker than Cuba’s, for example, or even more so, Turkey, which has pursued independent and active geopolitical changes on many fronts.
The development of sovereign Turkish vaccines is evidence that Ankara is now a first league country, one of the strongest countries in the world in terms of its scientific potential.
The very competition of vaccines testifies to the multipolarity of today’s world, where different centers are ready to offer their solutions to global problems. Given this context, initiatives such as COVAX with the participation of globalist capitalist structures such as the Bill and Melinda Gates Foundation prove that globalist networks will struggle to maintain their influence in a changing world.
Published at https://uwidata.com/13869-the-geopolitics-of-coronavirus-vaccines/